How To Determine Limiting Reactant And Excess Reactant
Approach 2 The The Product Method. The mass of product formed in a reaction depends upon the mass of the limiting reactant.

Need To Calculate The Limiting Reactant Of A Chemical Reaction Chemical Reactions Chemical Chemical Equation
Excess Reactant The reactant in a chemical reaction that remains when a reaction stops when the limiting reactant is completely consumed.

How to determine limiting reactant and excess reactant. Find out the number of moles of product with the help of a balanced chemical equation. To find the amount of remaining excess reactant subtract the mass of excess reactant consumed from the total mass of excess reactant given. Determine the limiting reagent if 100 g of each reagent are present at the beginning of the reaction.
Often it is straightforward to determine which reactant will be the limiting reactant but sometimes it takes a few extra steps. The key difference between limiting reactant and excess reactant is that the limiting reactant can limit the amount of final product produced whereas excess reactant has no effect on the amount of final product. Consider for example burning propane in a grill.
Limiting reagent is the reactant of a particular chemical reaction that limits the formation of the product. The key to recognizing which reactant is the limiting reactant is based on a mole-mass or mass-mass calculation. The propane and oxygen in the air combust to create heat and carbon dioxide.
Use the molar ratio from the equation to convert moles of O_2 from Step 3 to moles of NH_3 and then convert. In order to calculate the mass of the product first write the balanced equation and find out which reagent is in excess. Calculate theoretical yields of products formed in reactions that involve limiting reagents.
Using the mole ration. The reaction will stop when all of the limiting reactant is consumed. Find the limiting reagent by calculating and comparing the amount of product each reactant will produce.
Consider a chemical reaction. What is a Limiting Reagent. The limiting reactant or reagent can be determined by two methods.
NH_3 is the only other reactant so it is the excess reactant. Causey shows you step by step how to find the limiting reactant and excess reactant in a given reaction. Use the amount of limiting reactant to calculate the amount of product produced.
2H 2g O 2g 2H 2 O g Formation of water 2H 2. Let 1 mole of H 2 1 mole of O 2 were reacted to produce H20. The reactant that produces a larger amount of product is the excess reactant.
Identify the reactant which produces the least amount of product as limiting reactant. Limiting reagent and Excess Reagent Example. Using the product approach.
Balance the chemical equation for the chemical reaction. Differentiate between limiting reactant and excess. Chemical reaction equations give the ideal stoichiometric relationship among reactants and products.
The reactant reagent that gives the least amount of product is the limiting reactant the reactant reagent that gives the greater amount of product is the excess reactant. To find out a limiting reactant the steps involved are Calculate the number of moles from the given amount of reactant. In a balanced chemical equation no reactant is limiting or in excess.
Therefore the limiting reagent will decide the amount of product that is going to be formed after the completion of the reaction. Limiting Reactant The reactant in a chemical reaction that limits the amount of product that can be formed. The limiting and excess reagent can be determined as.
Finding the excess reactant. A reactant is a compound that is consumed during a chemical reactionA chemical reaction involves reactants some reactants in excess and some in limited amounts. However the reactants for a reaction in an experiment are not necessarily a.
Calculate the mass of excess reactant used up. Identify limiting and excess reactants O_2 is the limiting reactant since it gives the smaller amount of NO. You are obviously more likely to run out.
This is because no more product can form. Identify the excess reagent as well as how many grams of the excess reagent will remain when the. If necessary calculate how much is left in excess of the non-limiting reactant.
In order to find the limiting reagents excess reagents and products in this reaction you need to do the following. Thus product will be calculated by H 2 which is the limiting reactant. In most limiting reactant stoichiometry problems the real goal is to determine how much product could be formed from a particular reactant mixture.
Use stoichiometric calculation to determine excess and limiting reagents in a chemical reaction and explain why. This reactant is known as the limiting reactant. Whichever reactant gives the lesser amount of product is.
Excess Reagent Limiting Reagent Reactant Reagent. Limiting reagent is totally consumed during the reaction. The reactant that is left over is described as being in excess.
Excess reactant Limiting reactant As H 2 is consumed completely so it is limiting reactant while 2 moles of N 2 are left unreacted so it is substance in excess.
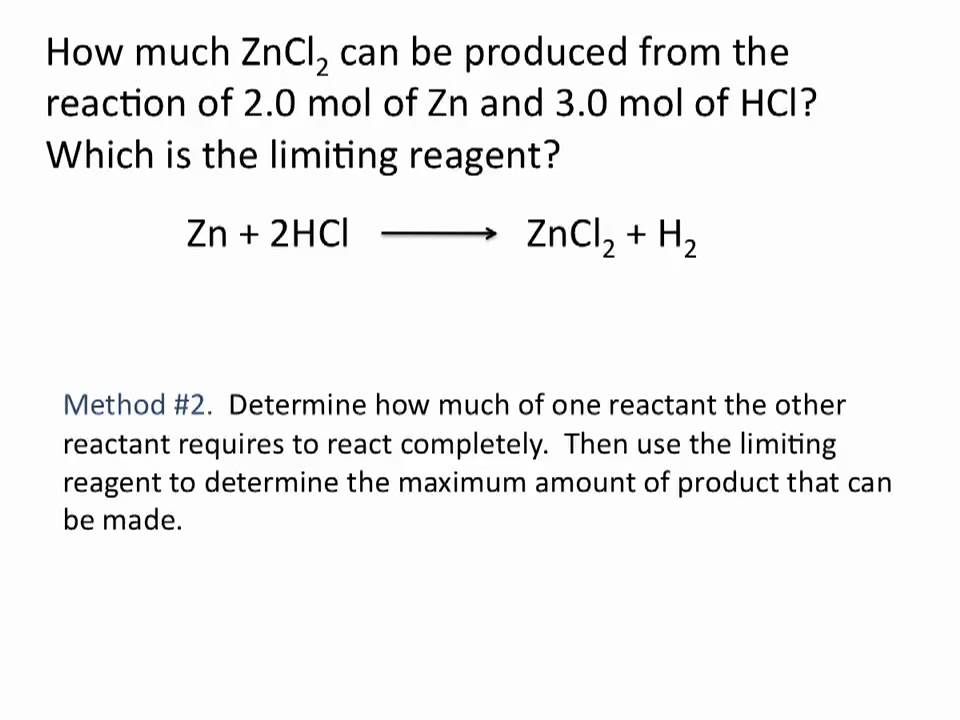
Limiting Reagent Chemistry Tutorial Youtube Chemistry Tutorial School

Limiting And Percent Yield A Chemistry Powerpoint Lesson Powerpoint Lesson Chemistry Lessons High School Science

Limiting Reactant Example Problem 1 Chemistry Problem Fuel Cells

An In Depth Introduction To Balancing Redox Equations Complete With A Step By Step Guide To Make Sure Nothing Is Missed In The P Redox Reactions Mcat Chemistry

Excess Reactant Unit 1 Stoichiometry Com Imagens Quimica
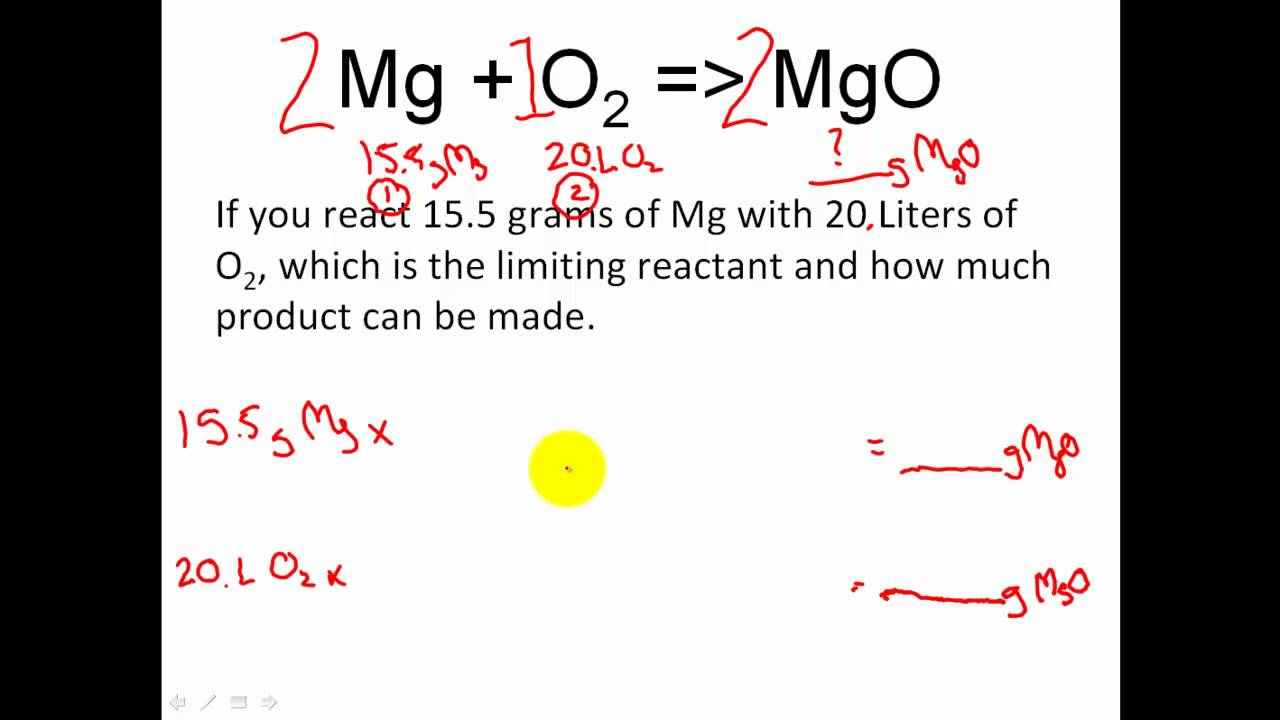
Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning Psychology Apologia Chemistry School Work

Skillbuilder 8 1 Unit 1 Stoichiometry Math Facts Addition Chemistry Lessons Chemistry Worksheets

General Chemistry Educational Infographics Limiting Reactants Video Chemistry Biology Science Classroom

Calculate Enthalpy Example Problems Solutions 4 Chemical Equation Problem And Solution Chemical Reactions

Limiting Reactant Scaffolded Notes By Threefourthsme Tpt Scaffolded Notes Covalent Bonding Teaching Style

Difference Between Limiting Reagent And Excess Reagent Comparison Summary Teaching Chemistry Biology Notes Chemistry Notes

Limiting Reactant Problems Chemistry Notes Chemistry Lecture Chemistry

Balance Different Types Of Reactions With A Simple Yet Efficient Method To Help Avoid All The Confusion M Chemistry Worksheets Teaching Chemistry Mcat Study

Limiting Reactant Scaffolded Notes By Threefourthsme Tpt Scaffolded Notes Lab Activities Color Activities

How To Calculate The Mass Of Excess Reactant Left Over In A Chemical Rea Chemistry Class Chemical Reactions Ap Chem

Mole Conversions Made Easy How To Convert Between Grams And Moles Youtube Scientific Skills Teaching Chemistry College Words

Limiting Reactant And Percent Yield Worksheet Bundle Science Lessons High School High School Science High School Science Class

How To Calculate Percent Yield In Chemistry Teaching Chemistry Chemistry Physical Chemistry

Limiting Reactant Practice Problem Science Sciencewithtylerdewitt Tylerdewitt Tutor Sciencehelp School Help Online School Chemistry
Post a Comment for "How To Determine Limiting Reactant And Excess Reactant"